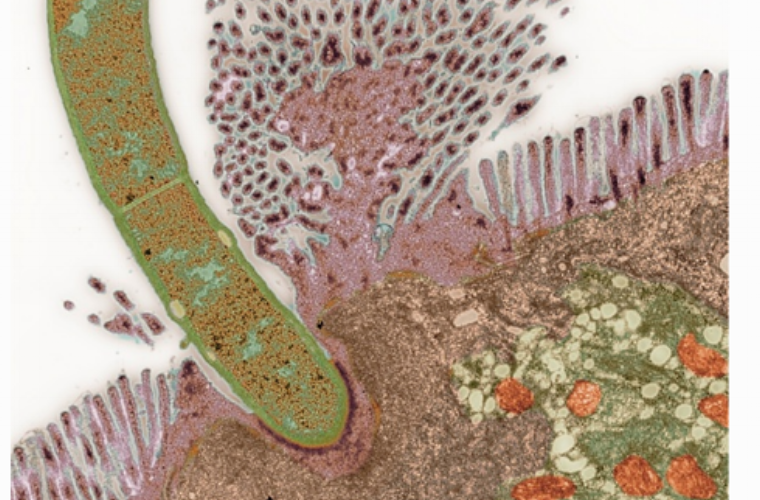
It is well recognized that the function of the intestinal immune system depends on the fine balance between effector, tolerogenic, and regulatory mechanisms. This balance is important not only in preventing disease, but also in providing flexibility and thus instructing the appropriate immune response. Dysregulation in any components of this homeostasis has been known to contribute to inflammatory bowel disease (IBD). Intestinal commensal bacteria are known to affect multiple immune mechanisms and mouse models of IBD depend on the presence of microbiota. However, the role of the composition of the microbiota has not been investigated, and whether different taxons of commensal bacteria activate specific branches of the immune system is not known. Th17 cells are a newly discovered helper T cell population proposed to have pro-inflammatory functions in IBD and other autoimmune diseases. At steady state Th17 cells are exclusively present in the small intestinal lamina propria where they co-exist in a well-regulated balance with Foxp3+ regulatory T cells (Treg). The role of different components of the intestinal microbiota in regulating this balance is currently unknown. We have discovered that the Th17:Treg ratio depends on the composition of the intestinal microbiota. Specific components of the commensal intestinal microbiota appear to induce Th17 cells that protect from infection with pathogenic microbes but can also have harmful inflammatory functions. We are studying bacterial taxons that specifically induce Th17 cell differentiation, examining the host signaling pathways that they engage, and investigating their involvement in both mucosal and systemic immunity. Information gathered from these studies will advance our understanding of the regulation of intestinal immune responses by commensal bacteria. The ultimate goal will be to provide knowledge for the rational design of therapeutic strategies for IBD and, potentially, other autoimmune diseases, based on modulating the composition or function of the intestinal microbiota.
In related studies, we are investigating the roles of transcriptional and post-transcriptional regulation in the differentiation program of Th17 cells. A major effort is focused on the function of the orphan nuclear receptor RORγt, the central transcriptional coordinator for the Th17 program. We are characterizing the transcriptional regulatory targets for this factor and for a series of other transcription factors that are required for expression of the Th17 cytokines (IL-17, IL-17F, IL-22) and Th17 surface molecules. We are also using genetic and biochemical approaches to identify a natural ligand for RORγt as well as inhibitors that can be employed in anti-inflammatory therapies. Another interest is the role of non-coding RNAs in the differentiation of Th17 and Treg cells. We have found that inhibition of the micro-RNA biosynthetic machinery results in defective differentiation of Treg cells and in exaggerated expression of inflammatory cytokines. We are comparing the different functions of the RNaseIII enzymes Drosha and Dicer in discrete developmental stages in T cells and other cell types.
Recent Publication
Xu, M., Pokrovskii, M., Ding, Y., Yi, R., Au, C., Harrison, O.J., Galan, C., Belkaid, Y., Bonneau, R., Littman, D.R. (2018) c-Maf-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature. 2018 Feb 15;554(7692):373-377. [PubMed]
Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, Littman DR. (2015) An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell. 2015 Oct 8;163(2):381-93 [Pubmed]
Santori FR, Huang P, van de Pavert SA, Douglass EF Jr, Leaver DJ, Haubrich BA, Keber R, Lorbek G, Konijn T, Rosales BN, Rozman D, Horvat S, Rahier A, Mebius RE, Rastinejad F, Nes WD, Littman DR. (2015) Identification of natural RORγ ligands that regulate the development of lymphoid cells. Cell Metab. 21(2):286-97. [Pubmed]
Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR. (2014) CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Ex Med. 211(8):1571-83. [Pubmed]
Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. (2014) Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 510(7503):152-6. [Pubmed]
Kruglov, A.A., Grivennikov, S.I., Kuprash, D.V., Winsauer, C., Prepens, S., Seleznik, G.M., Eberl, G., Littman, D.R., Heikenwalder, M., Tumanov, A.V., Nedospasov, S.A. (2013) Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science. 342(6163):1243-6.
Scher, J.U., Sczesnak, A., Longman, R.S., Segata, N., Ubeda, C., Bielski, C., Rostron, T., Cerundolo, V., Pamer, E.G., Abramson, S.B., Huttenhower, C., Littman, D.R. (2013) Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2:e01202. PMC3816614.
Kim, S.V., Xiang, W.V., Kwak, C., Yang, Y., Lin, X.W., Ota, M., Sarpel, U., Rifkin, D.B., Xu, R., & Littman, D.R. (2013) GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. Published online May 9 ahead of print.
Diehl, G.E., Longman, R.S., Zhang, J.X, Breart, B., Galan, C., Cuesta, A., Schwab, S.R. & Littman, D.R. (2013) Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature, 494:116-20. PMC3711636.
Ciofani, M., Madar, A., Galan, C., Sellars, M., Mace, K., Pauli, F., Agarwal, A., Huang, W., Parkurst, C.N., Muratet, M., Newberry, K.M., Meadows, S., Greenfield, A., Yang, Y., Jain, P., Kirigin, F.F., Birchmeier, C., Wagner, E.F., Murphy, K.M., Myers, R.M., Bonneau, R. & Littman, D.R. (2012) A validated regulatory network for Th17 cell specification. Cell, 151:289-303. PMC3503487.
Weiss, J.M., Bilate, A.M., Gobert, M., Ding, Y., Curotto de Lafaille, M.A., Parkhurst, C.N., Xiong, H., Dolpady, J., Frey, A.B., Ruocco, M.G., Yang, Y., Floess, S., Huehn, J., Oh, S., Li, M.O., Niec, R.E., Rudensky, A.Y., Dustin, M.L., Littman, D.R., Lafaille, J.J. (2012) Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209:1723-42. PMC3457733.
Glasmacher, E., Agrawal, S., Chang, A.B., Murphy, T.L., Zeng, W., Vander Lugt, B., Khan, A.A., Ciofani, M., Spooner, C.J., Rutz, S., Hackney, J., Nurieva, R., Escalante, C.R., Ouyang, W., Littman, D.R., Murphy, K.M., Singh, H. (2012) A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science. 338:975-80.
Fulton, L.M., Carlson, M.J., Coghill, J.M., Ott, L.E., West, M.L., Panoskaltsis-Mortari, A., Littman, D.R., Blazar, B.R. & Serody, J.S. (2012) Attenuation of Acute Graft-versus-Host Disease in the Absence of the Transcription Factor RORγt. J. Immunol. 189(4):1765-72. PMC3411855.
Huh, J.R., & Littman, D.R. (2012) Small molecule inhibitors of RORγt: Targeting Th17 cells and other applications. Eur. J. Immunol. 42(9):2232-7.
Hooper, L.V., Littman, D.R. & Macpherson, A.J. (2012) Interactions between the microbiota and the immune system. Science 336(6086), 1268-73.
Scher, J.U., Ubeda, C., Equinda, M., Khanin, R., Buischi, Y, Viale, A., Lipuma, L., Attur, M., Pillinger, M.H., Weissmann, G., Littman, D.R., Pamer, E.G., Bretz, W.A. & Abramson, S.B. (2012) Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. Epub 2012 May 10. PMC3428472.
Kinnebrew, M.A., Buffie, C.G., Diehl, G.E., Zenewicz, L.A., Leiner, I., Hohl, T.M., Flavell, R.A., Littman, D.R. & Pamer, E.G. (2012) Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36(2), 276-87. PMC3288454.
Honda, K. & Littman, D.R. (2012) The microbiome in infectious disease and inflammation. Ann. Rev. Immunol. 30, 759-95.
Ivanov, I.I. & Littman, D.R. (2011) Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 14(1):106-14. PMC3123735.
Hovhannisyan, Z., Treatman, J., Littman, D.R. & Mayer, L. (2011) Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 140(3), 957-65. PMC3049831.
Sujino, T., Kanai, T., Ono, Y., Mikami, Y., Hayashi, A., Doi, T., Matsuoka, K., Hisamatsu, T., Takaishi, H., Ogata, H., Yoshimura, A., Littman, D.R. & Hibi, T. (2011) Regulatory T Cells Suppress the Development of Colitis, Blocking Differentiation of T-helper 17 Into Alternative T-Helper 1 Cells. Gastroenterology 141(3), 1014-23.
Sczesnak, A., Segata, N., Qin, X., Gevers, D., Petrosino, J.F., Huttenhower, C., Littman, D.R. and Ivanov, I.I. (2011) The genome of Th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell Host Microbe 10(3), 260-72. PMC3209701.
Littman, D.R. & Pamer, E.G. (2011) Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10(4), 311-23. PMC3202012.